Isotop talium
| |||||||||||||||||||||||||||||||
| Berat atom standar Ar°(Tl) |
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
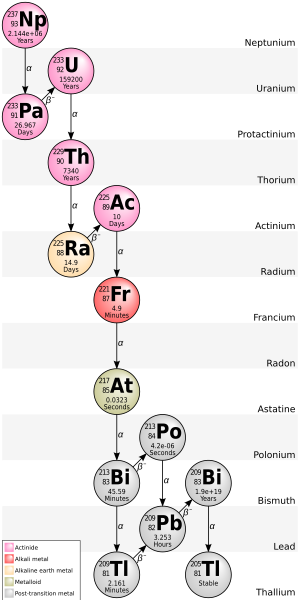
Talium (81Tl) memiliki 41 isotop dengan massa atom berkisar antara 176 hingga 216. Talium memiliki dua isotop stabil, 203Tl dan 205Tl. 204Tl adalah radioisotop talium yang paling stabil dengan waktu paruh 3,78 tahun. 207Tl, dengan waktu paruh 4,77 menit, memiliki waktu paruh terpanjang dari semua radioisotop talium alami. Semua isotop talium bersifat radioaktif atau stabil secara pengamatan, artinya mereka diprediksi bersifat radioaktif tetapi tidak ada peluruhan aktual yang teramati.
202Tl (waktu paruh 12,23 hari) dapat dibuat dalam siklotron sedangkan 204Tl (waktu paruh 3,78 tahun) dibuat dengan aktivasi neutron dari talium stabil dalam reaktor nuklir.
Dalam keadaan terionisasi penuh, isotop 205Tl dapat menjadi radioaktif, meluruh melalui peluruhan beta menjadi 205Pb, tetapi 203Tl tetap stabil.
205Tl merupakan produk peluruhan dari 209Bi, sebuah isotop yang pernah dianggap stabil tetapi sekarang diketahui mengalami peluruhan alfa dengan waktu paruh yang sangat panjang, yaitu 2,01×1019 tahun.205Tl berada di ujung rantai peluruhan deret neptunium.
Daftar isotop
| Nuklida |
Nama historis |
Z | N |
Massa isotop (Da) |
Waktu paruh |
Mode peluruhan |
Isotop anak |
Spin dan paritas |
Kelimpahan alami (fraksi mol) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energi eksitasi | Proporsi normal | Rentang variasi | |||||||||||||||||
| 176Tl | 81 | 95 | 176,00059(21)# | 5,2(+30−14) mdtk | (3−, 4−, 5−) | ||||||||||||||
| 177Tl | 81 | 96 | 176,996427(27) | 18(5) mdtk | p | 176Hg | (1/2+) | ||||||||||||
| α (langka) | 173Au | ||||||||||||||||||
| 177mTl | 807(18) keV | 230(40) μs | p | 176Hg | (11/2−) | ||||||||||||||
| α | 173Au | ||||||||||||||||||
| 178Tl | 81 | 97 | 177,99490(12)# | 255(10) mdtk | α | 174Au | |||||||||||||
| p (langka) | 177Hg | ||||||||||||||||||
| 179Tl | 81 | 98 | 178,99109(5) | 270(30) mdtk | α | 175Au | (1/2+) | ||||||||||||
| p (langka) | 178Hg | ||||||||||||||||||
| 179mTl | 860(30)# keV | 1,60(16) mdtk | α | 175Au | (9/2−) | ||||||||||||||
| IT (langka) | 179Tl | ||||||||||||||||||
| 180Tl | 81 | 99 | 179,98991(13)# | 1,5(2) dtk | α (75%) | 176Au | |||||||||||||
| β+ (25%) | 180Hg | ||||||||||||||||||
| EC, fisi (10−4%) | 100Ru, 80Kr | ||||||||||||||||||
| 181Tl | 81 | 100 | 180,986257(10) | 3,2(3) dtk | α | 177Au | 1/2+# | ||||||||||||
| β+ | 181Hg | ||||||||||||||||||
| 181mTl | 857(29) keV | 1,7(4) mdtk | α | 177Au | 9/2−# | ||||||||||||||
| β+ | 181Hg | ||||||||||||||||||
| 182Tl | 81 | 101 | 181,98567(8) | 2,0(3) dtk | β+ (96%) | 182Hg | 2−# | ||||||||||||
| α (4%) | 178Au | ||||||||||||||||||
| 182m1Tl | 100(100)# keV | 2,9(5) dtk | α | 178Au | (7+) | ||||||||||||||
| β+ (langka) | 182Hg | ||||||||||||||||||
| 182m2Tl | 600(140)# keV | 10− | |||||||||||||||||
| 183Tl | 81 | 102 | 182,982193(10) | 6,9(7) dtk | β+ (98%) | 183Hg | 1/2+# | ||||||||||||
| α (2%) | 179Au | ||||||||||||||||||
| 183m1Tl | 630(17) keV | 53,3(3) mdtk | IT (99,99%) | 183Tl | 9/2−# | ||||||||||||||
| α (0,01%) | 179Au | ||||||||||||||||||
| 183m2Tl | 976,8(3) keV | 1,48(10) μdtk | (13/2+) | ||||||||||||||||
| 184Tl | 81 | 103 | 183,98187(5) | 9,7(6) dtk | β+ | 184Hg | 2−# | ||||||||||||
| 184m1Tl | 100(100)# keV | 10# dtk | β+ (97,9%) | 184Hg | 7+# | ||||||||||||||
| α (2,1%) | 180Au | ||||||||||||||||||
| 184m2Tl | 500(140)# keV | 47,1 mdtk | IT (99,911%) | (10−) | |||||||||||||||
| α (0,089%) | 180Au | ||||||||||||||||||
| 185Tl | 81 | 104 | 184,97879(6) | 19,5(5) dtk | α | 181Au | 1/2+# | ||||||||||||
| β+ | 185Hg | ||||||||||||||||||
| 185mTl | 452,8(20) keV | 1,93(8) dtk | IT (99,99%) | 185Tl | 9/2−# | ||||||||||||||
| α (0,01%) | 181Au | ||||||||||||||||||
| β+ | 185Hg | ||||||||||||||||||
| 186Tl | 81 | 105 | 185,97833(20) | 40# dtk | β+ | 186Hg | (2−) | ||||||||||||
| α (0,006%) | 182Au | ||||||||||||||||||
| 186m1Tl | 320(180) keV | 27,5(10) dtk | β+ | 186Hg | (7+) | ||||||||||||||
| 186m2Tl | 690(180) keV | 2,9(2) dtk | (10−) | ||||||||||||||||
| 187Tl | 81 | 106 | 186,975906(9) | ~51 dtk | β+ | 187Hg | (1/2+) | ||||||||||||
| α (langka) | 183Au | ||||||||||||||||||
| 187mTl | 335(3) keV | 15,60(12) dtk | α | 183Au | (9/2−) | ||||||||||||||
| IT | 187Tl | ||||||||||||||||||
| β+ | 187Hg | ||||||||||||||||||
| 188Tl | 81 | 107 | 187,97601(4) | 71(2) dtk | β+ | 188Hg | (2−) | ||||||||||||
| 188m1Tl | 40(30) keV | 71(1) dtk | β+ | 188Hg | (7+) | ||||||||||||||
| 188m2Tl | 310(30) keV | 41(4) mdtk | (9−) | ||||||||||||||||
| 189Tl | 81 | 108 | 188,973588(12) | 2,3(2) mnt | β+ | 189Hg | (1/2+) | ||||||||||||
| 189mTl | 257,6(13) keV | 1,4(1) mnt | β+ (96%) | 189Hg | (9/2−) | ||||||||||||||
| IT (4%) | 189Tl | ||||||||||||||||||
| 190Tl | 81 | 109 | 189,97388(5) | 2,6(3) mnt | β+ | 190Hg | 2(−) | ||||||||||||
| 190m1Tl | 130(90)# keV | 3,7(3) mnt | β+ | 190Hg | 7(+#) | ||||||||||||||
| 190m2Tl | 290(70)# keV | 750(40) μdtk | (8−) | ||||||||||||||||
| 190m3Tl | 410(70)# keV | >1 μdtk | 9− | ||||||||||||||||
| 191Tl | 81 | 110 | 190,971786(8) | 20# mnt | β+ | 191Hg | (1/2+) | ||||||||||||
| 191mTl | 297(7) keV | 5,22(16) mnt | β+ | 191Hg | 9/2(−) | ||||||||||||||
| 192Tl | 81 | 111 | 191,97223(3) | 9,6(4) mnt | β+ | 192Hg | (2−) | ||||||||||||
| 192m1Tl | 160(50) keV | 10,8(2) mnt | β+ | 192Hg | (7+) | ||||||||||||||
| 192m2Tl | 407(54) keV | 296(5) ndtk | (8−) | ||||||||||||||||
| 193Tl | 81 | 112 | 192,97067(12) | 21,6(8) mnt | β+ | 193Hg | 1/2(+#) | ||||||||||||
| 193mTl | 369(4) keV | 2,11(15) mnt | IT (75%) | 193Tl | 9/2− | ||||||||||||||
| β+ (25%) | 193Hg | ||||||||||||||||||
| 194Tl | 81 | 113 | 193,97120(15) | 33,0(5) mnt | β+ | 194Hg | 2− | ||||||||||||
| α (10−7%) | 190Au | ||||||||||||||||||
| 194mTl | 300(200)# keV | 32,8(2) mnt | β+ | 194Hg | (7+) | ||||||||||||||
| 195Tl | 81 | 114 | 194,969774(15) | 1,16(5) jam | β+ | 195Hg | 1/2+ | ||||||||||||
| 195mTl | 482,63(17) keV | 3,6(4) dtk | IT | 195Tl | 9/2− | ||||||||||||||
| 196Tl | 81 | 115 | 195,970481(13) | 1,84(3) jam | β+ | 196Hg | 2− | ||||||||||||
| 196mTl | 394,2(5) keV | 1,41(2) jam | β+ (95,5%) | 196Hg | (7+) | ||||||||||||||
| IT (4,5%) | 196Tl | ||||||||||||||||||
| 197Tl | 81 | 116 | 196,969575(18) | 2,84(4) jam | β+ | 197Hg | 1/2+ | ||||||||||||
| 197mTl | 608,22(8) keV | 540(10) mdtk | IT | 197Tl | 9/2− | ||||||||||||||
| 198Tl | 81 | 117 | 197,97048(9) | 5,3(5) jam | β+ | 198Hg | 2− | ||||||||||||
| 198m1Tl | 543,5(4) keV | 1,87(3) jam | β+ (54%) | 198Hg | 7+ | ||||||||||||||
| IT (46%) | 198Tl | ||||||||||||||||||
| 198m2Tl | 687,2(5) keV | 150(40) ndtk | (5+) | ||||||||||||||||
| 198m3Tl | 742,3(4) keV | 32,1(10) mdtk | (10−)# | ||||||||||||||||
| 199Tl | 81 | 118 | 198,96988(3) | 7,42(8) jam | β+ | 199Hg | 1/2+ | ||||||||||||
| 199mTl | 749,7(3) keV | 28,4(2) mdtk | IT | 199Tl | 9/2− | ||||||||||||||
| 200Tl | 81 | 119 | 199,970963(6) | 26,1(1) jam | β+ | 200Hg | 2− | ||||||||||||
| 200m1Tl | 753,6(2) keV | 34,3(10) mdtk | IT | 200Tl | 7+ | ||||||||||||||
| 200m2Tl | 762,0(2) keV | 0,33(5) μdtk | 5+ | ||||||||||||||||
| 201Tl | 81 | 120 | 200,970819(16) | 72,912(17) jam | EC | 201Hg | 1/2+ | ||||||||||||
| 201mTl | 919,50(9) keV | 2,035(7) mdtk | IT | 201Tl | (9/2−) | ||||||||||||||
| 202Tl | 81 | 121 | 201,972106(16) | 12,23(2) hri | β+ | 202Hg | 2− | ||||||||||||
| 202mTl | 950,19(10) keV | 572(7) μdtk | 7+ | ||||||||||||||||
| 203Tl | 81 | 122 | 202,9723442(14) | Stabil Secara Pengamatan | 1/2+ | 0,2952(1) | 0,29494–0,29528 | ||||||||||||
| 203mTl | 3400(300) keV | 7,7(5) μdtk | (25/2+) | ||||||||||||||||
| 204Tl | 81 | 123 | 203,9738635(13) | 3,78(2) thn | β− (97,1%) | 204Pb | 2− | ||||||||||||
| EC (2,9%) | 204Hg | ||||||||||||||||||
| 204m1Tl | 1104,0(4) keV | 63(2) μdtk | (7)+ | ||||||||||||||||
| 204m2Tl | 2500(500) keV | 2,6(2) μdtk | (12−) | ||||||||||||||||
| 204m3Tl | 3500(500) keV | 1,6(2) μdtk | (20+) | ||||||||||||||||
| 205Tl | 81 | 124 | 204,9744275(14) | Stabil Secara Pengamatan | 1/2+ | 0,7048(1) | 0,70472–0,70506 | ||||||||||||
| 205m1Tl | 3290,63(17) keV | 2,6(2) μdtk | 25/2+ | ||||||||||||||||
| 205m2Tl | 4835,6(15) keV | 235(10) ndtk | (35/2–) | ||||||||||||||||
| 206Tl | Radium E | 81 | 125 | 205,9761103(15) | 4,200(17) mnt | β− | 206Pb | 0− | Renik | ||||||||||
| 206mTl | 2643,11(19) keV | 3,74(3) mnt | IT | 206Tl | (12–) | ||||||||||||||
| 207Tl | Aktinium C | 81 | 126 | 206,977419(6) | 4,77(2) mnt | β− | 207Pb | 1/2+ | Renik | ||||||||||
| 207mTl | 1348,1(3) keV | 1,33(11) dtk | IT (99,9%) | 207Tl | 11/2– | ||||||||||||||
| β− (0,1%) | 207Pb | ||||||||||||||||||
| 208Tl | Torium C" | 81 | 127 | 207,9820187(21) | 3,053(4) mnt | β− | 208Pb | 5+ | Renik | ||||||||||
| 209Tl | 81 | 128 | 208,985359(8) | 2,161(7) mnt | β− | 209Pb | 1/2+ | Renik | |||||||||||
| 210Tl | Radium C″ | 81 | 129 | 209,990074(12) | 1,30(3) mnt | β− (99,991%) | 210Pb | (5+)# | Renik | ||||||||||
| β−, n (0,009%) | 209Pb | ||||||||||||||||||
| 211Tl | 81 | 130 | 210,993480(50) | 80(16) dtk | β− (97,8%) | 211Pb | 1/2+ | ||||||||||||
| β−, n (2,2%) | 210Pb | ||||||||||||||||||
| 212Tl | 81 | 131 | 211,998340(220)# | 31(8) dtk | β− (98,2%) | 212Pb | (5+) | ||||||||||||
| β−, n (1,8%) | 211Pb | ||||||||||||||||||
| 213Tl | 81 | 132 | 213,001915(29) | 24(4) dtk | β− (92,4%) | 213Pb | 1/2+ | ||||||||||||
| β−, n (7,6%) | 212Pb | ||||||||||||||||||
| 214Tl | 81 | 133 | 214,006940(210)# | 11(2) dtk | β− (66%) | 214Pb | 5+# | ||||||||||||
| β−, n (34%) | 213Pb | ||||||||||||||||||
| 215Tl | 81 | 134 | 215,010640(320)# | 10(4) dtk | β− (95,4%) | 215Pb | 1/2+# | ||||||||||||
| β−, n (4,6%) | 214Pb | ||||||||||||||||||
| 216Tl | 81 | 135 | 216,015800(320)# | 6(3) dtk | β− | 216Pb | 5+# | ||||||||||||
| β−, n (<11,5%) | 215Pb | ||||||||||||||||||
| Header & footer tabel ini: | |||||||||||||||||||
Talium-201
Talium-201 (201Tl) adalah sebuah radioisotop sintetis talium. Ia memiliki waktu paruh 73 jam dan meluruh dengan menangkap elektron, memancarkan sinar-X (~70–80 keV), dan foton 135 dan 167 keV dalam kelimpahan total 10%.201Tl disintesis oleh aktivasi neutron talium stabil dalam reaktor nuklir, atau dengan reaksi nuklir 203Tl(p, 3n)201Pb di dalam siklotron, karena 201Pb secara alami meluruh menjadi 201Tl sesudahnya. Ia merupakan radiofarmasi, karena memiliki karakteristik pencitraan yang baik tanpa dosis radiasi pasien yang berlebihan. Ia juga merupakan isotop paling populer yang digunakan untuk iskemia koroner inti talium.
- Massa isotop dari:
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- Komposisi isotop dan massa atom standar dari:
-
de Laeter, John Robert; Böhlke, John Karl; De Bièvre, Paul; Hidaka, Hiroshi; Peiser, H. Steffen; Rosman, Kevin J. R.; Taylor, Philip D. P. (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683
 .
.
-
Wieser, Michael E. (2006). "Atomic weights of the elements 2005 (IUPAC Technical Report)". Pure and Applied Chemistry. 78 (11): 2051–2066. doi:10.1351/pac200678112051
 .
.
-
de Laeter, John Robert; Böhlke, John Karl; De Bièvre, Paul; Hidaka, Hiroshi; Peiser, H. Steffen; Rosman, Kevin J. R.; Taylor, Philip D. P. (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683
- "News & Notices: Standard Atomic Weights Revised". International Union of Pure and Applied Chemistry. 19 Oktober 2005.
- Data waktu paruh, spin, dan isomer dipilih dari sumber-sumber berikut.
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- National Nuclear Data Center. "NuDat 2.x database". Laboratorium Nasional Brookhaven.
- Holden, Norman E. (2004). "11. Table of the Isotopes". Dalam Lide, David R. CRC Handbook of Chemistry and Physics (edisi ke-85). Boca Raton, Florida: CRC Press. ISBN 978-0-8493-0485-9.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||